Draw and Describe With Words the Unit Cells for Face-centered
The conventional unit cell chosen is usually bigger than the primitive cell in favor of preserving the symmetry of the Bravais lattice. The hexagonal closest packed HCP has a coordination number of 12 and contains 6 atoms per unit cell.

Cbse Class 12 Chemistry Notes Chapter 1 The Solid State
DC - A special type of face-centered cubic crystal structure found in carbon silicon α-Sn and other covalently bonded materials.
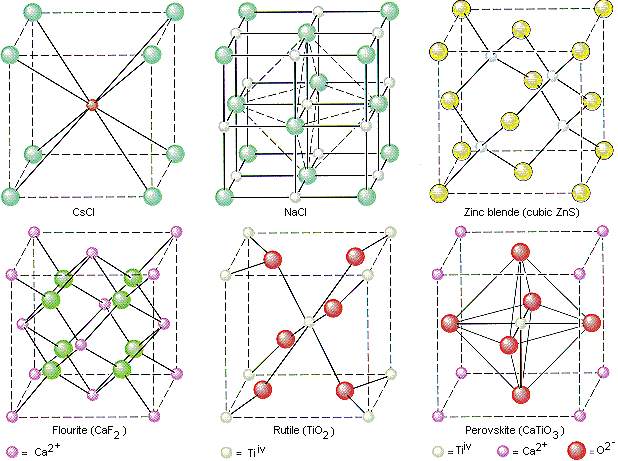
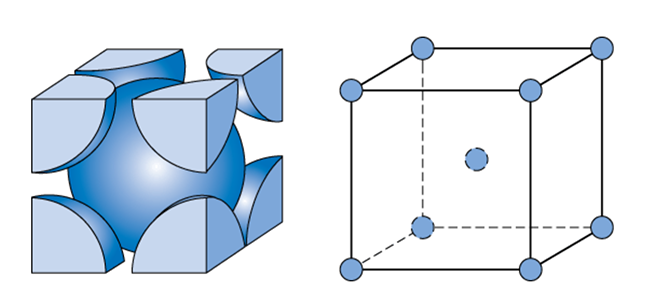
. The simplest repeating arrangement of a lattice point in 3D is called the unit cell. A face-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube has a fraction of an atom with six additional full atoms positioned at the center of each cube face. Problems 1 2 and 3 in Chapter 1.
3 4 12 a. Wish to draw the plane within the unit cell let s move the origin 1 in the x-direction to 1 0 0. The cubic crystal structure for example consists of three distinct unit cell types.
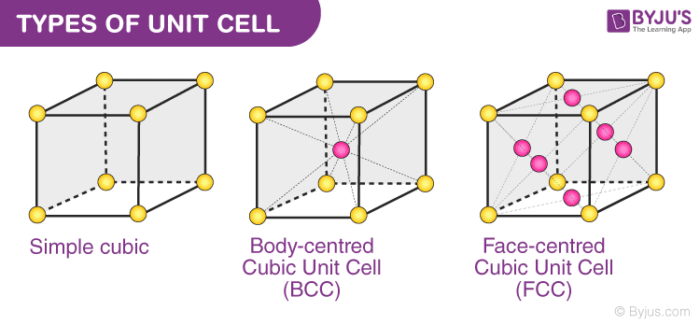
Simple Cubic Ba. It is very cumbersome to draw entire lattices in 3D so some small portion of the lattice having full symmetry of the lattice is usually drawn. The atoms at the corner of the cube are shared with eight.
Describe and make a drawing of the dislocation. Face-Centered Cubic FCC Na a000 Cl a 1 200. B note the.
Therefore we can say that 68 volume of the unit cell of BCC is occupied by atoms and remaining 32 volume is vacant. Draw unit cells for. Body Centered Cubic Specific Heat Capacity Of Aluminum Body Centered Cubic Unit Cell Normal Boiling Point Face Centered Cubic Unit Cell TERMS IN THIS SET 30 Consider the following two substances and their vapor pressures at 298 K.
For questions 3 4 no calculations are involved. Substance Vapor pressure mmHg A 275 B 459 Based on this information compare the characteristics of the two. Number of atoms per unit cell2.
NB I have distorted the cube to make the later stages of the task a little more easy. 3 4 8 a. The number of atoms present in a unit cell 2 atoms.
Problems 3 4 and 5. The volume of the primitive unit cell is equal to the volume of the conventional unit cell divided by the number of sites. Face-Centered Cubic FCC Ca a000 F.
Packing Factor Number of atoms present per unit cell x Volume of atom Volume of the Unit Cell. So the first thing to do is to draw a cube shaped box. Lattice has one additional point at the center of the cell and a face-centered lattice has six additional points one on each side.
21 o Miller-indices - A shorthand notation to describe. Ventional cubic unit cell of the snicture. This small portion when repeated can generate the whole lattice and is called the unit cell and it could be larger than the primitive cell Unit Cell.
The diamond cubic cell belongs to space group 227 or Strukturbericht A4 and Pearson symbol cF8. A unit cell contains one constituent particle present at the centre of each face along with eight particles at its corners. C diamond is the prototype for DC.
The face-centered cubic unit cell contains a single octahedral hole within itself but octahedral holes shared with adjacent cells exist at the centers of each edge. A a a Unit cell of a cubic lattice a1 a2 a3 a1. Nnumber of atomsunit cell volume 3 a.
Height of unit cell. 1 4 F. The Unit Cell.
Volume area of base height. The Diamond Cubic DC unit cell can be imagined as a cube with an atom on each corner each face and the ¼ ¼ ¼ ¾ ¾ ¼ ¼ ¼ ¾ and ¼ ¾ ¾ positions. Derive the relationships between unit cell edge length and atomic radius for face-centered cubic and body-centered cubic crystal structures.
The smallest replicating portion of a crystal lattice is a unit cell. This implies face-centered tetragonal lattice doesnt exist. Unit cells exist in many types.
Face-centered cubic FCC or cF is the name given to a type of atom arrangement found in nature. Each of these twelve edge-located sites is shared with four adjacent cells and thus contributes 12 ¼ 3 atoms to the cell. Specify which are close packed meaning that the atoms are arranged and packed together as closely as possible.
Nnumber of atomsunit cell volume 2 a. DC is a famously strong crystal structure and is the structure of diamond. A unit cell is the smallest representation of an entire crystal.
1 a 1 4. A unit cell is a volume when translated through some subset of the vectors of a Bravais lattice can fill up the whole space without voids or overlapping with itself. Refer to your chemistry text.
Mechanical Engineering questions and answers. This turn out to be a body-centered tetragonal unit cell. First view of the cube showing a cube as a solid object.
Packing Fraction Show that the maximum proportion of the available volume which may be filled by hard spheres arranged on various laffices is. Then we can. Note that in all the non-simple lattices the unit cells are non-primitive.
Relating to or being a crystal space lattice in which each cubic unit cell has an atom at the center and at the corners of each face compare body-centered. Crystal Structure 3 Unit cell and lattice constants. Nnumber of atomsunit cell volume 2 a.
If we draw face centered unit cell in tetragonal lattice then the resulting cell will have half of the volume to original lattice. How many atoms are in the unit cell. We start by looking at the size and shape of the unit cell the unit cell is cubic and the length of one side is 3615 Å.
Unit cell and its types. Thus the Packing Density is 68. No of atoms present in this unit cell 8 x 18 1 2 Each Corner atom contributes 18 th portion to the unit cell Body centered atom is 1 iii Face-centred cubic unit cell FCC.
The face-centered cubic FCC has a coordination number of 12 and contains 4 atoms per unit cell. Metallic crystal structures Draw and describe with words the unit cells for face-centered cubic FCC body-centered cubic BCC and hexagonal close packed HCP crystal structures. In your own words describe the face-centered cubic unit cell in such a way that someone reading only your description would be able to make a reasonable sketch of what it looks like.
1 face-centered cubic 2 body-centered cubic 3 hexagonal close-packed crystal structures. It has 4-fold rotational symmetry.

Unit Cell Chemistry Simple Cubic Body Centered Cubic Face Centered Cubic Crystal Lattice Structu Youtube

Cbse Class 12 Chemistry Notes Chapter 1 The Solid State

Unit Cells Chemistry For Non Majors
Face Centered Cubic Fcc Unit Cell Materials Science Engineering
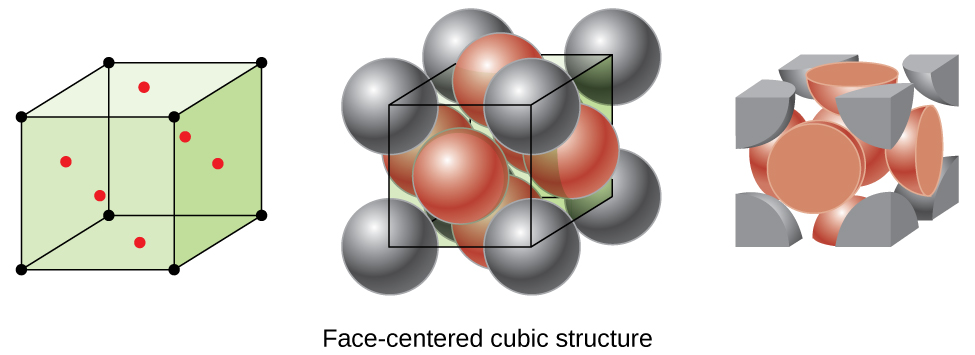
4 1 Unit Cells Chemistry Libretexts
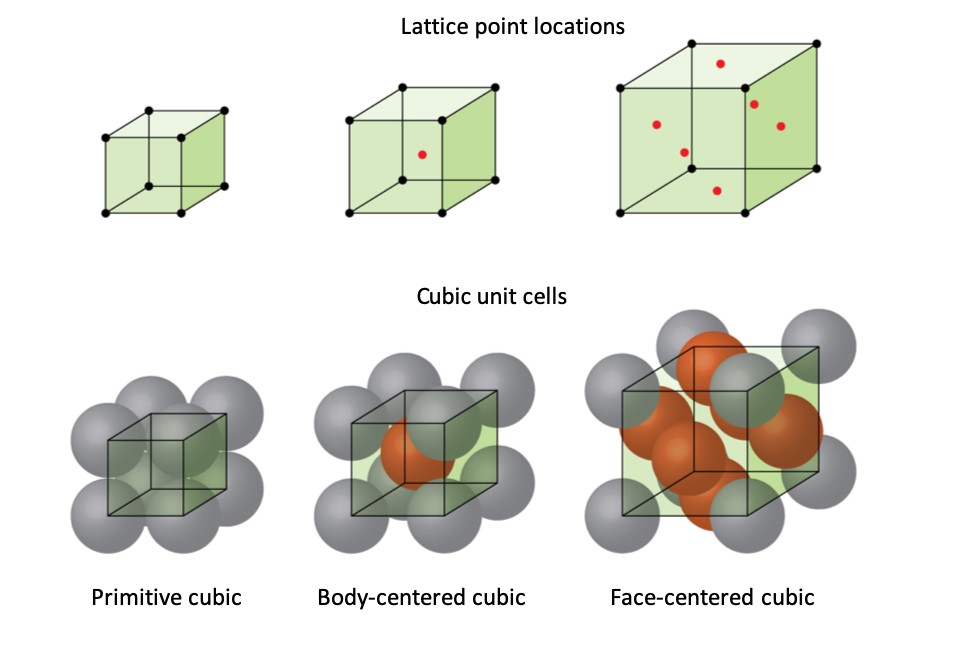
Types Of Unit Cells Body Centered Cubic And Face Centered Cubic M11q5 Uw Madison Chemistry 103 104 Resource Book

Unit Cell Overview Examples Expii
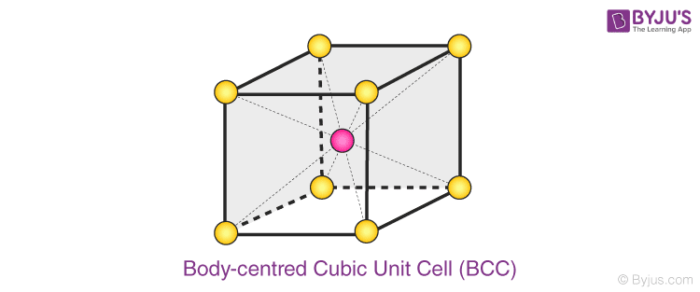
What Is A Unit Cell Definition Types Of Unit Cell Primitive Unit Cell Bcc Fcc Volume Of Hcp Unit Cell
Body Centered Cubic Structure Bcc Matse 81 Materials In Today S World

5 Unit Cells For Simple Cubic Body Centered Cubic Bcc And Download Scientific Diagram
Face Centered Cubic Fcc Unit Cell Materials Science Engineering
What Is A Unit Cell Definition Types Of Unit Cell Primitive Unit Cell Bcc Fcc Volume Of Hcp Unit Cell

Unit Cells Chemistry For Non Majors

What Is A Unit Cell Definition Types Of Unit Cell Primitive Unit Cell Bcc Fcc Volume Of Hcp Unit Cell

Types Of Unit Cells Definition Examples Diagrams
What Is The Difference Between Fcc And Bcc Crystal Structure Properties Interstitial Sites And Examples Materials Science Engineering
Face Centered Cubic Fcc Unit Cell Materials Science Engineering

What Is A Unit Cell Definition Types Of Unit Cell Primitive Unit Cell Bcc Fcc Volume Of Hcp Unit Cell
Comments
Post a Comment